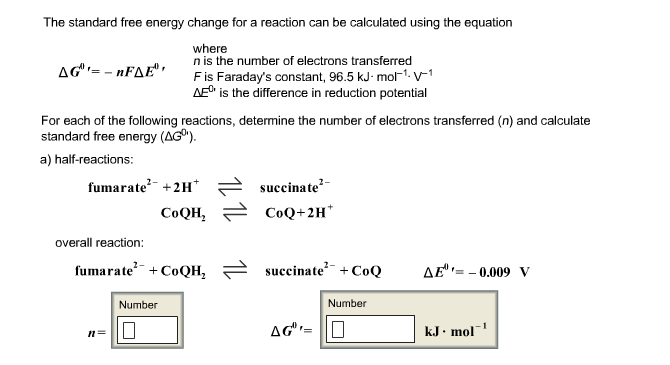
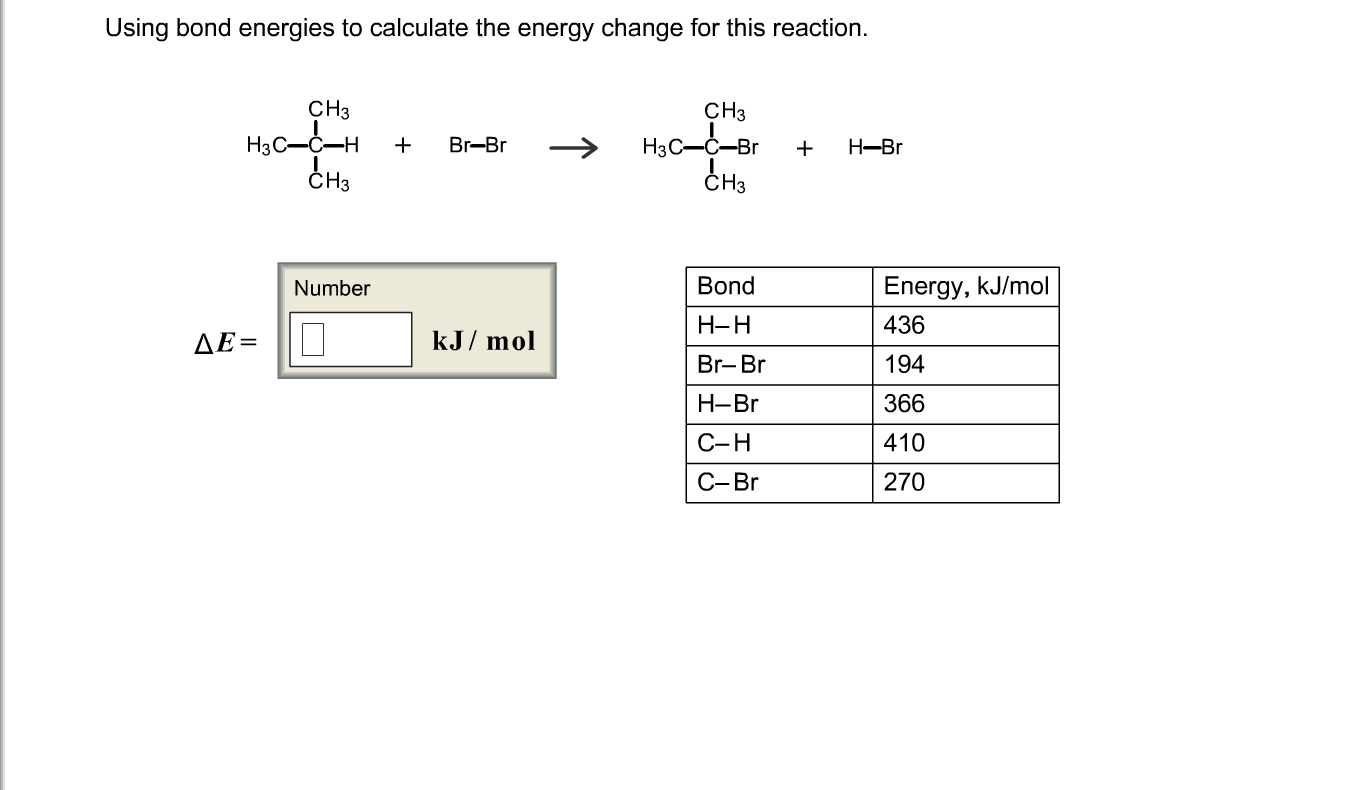
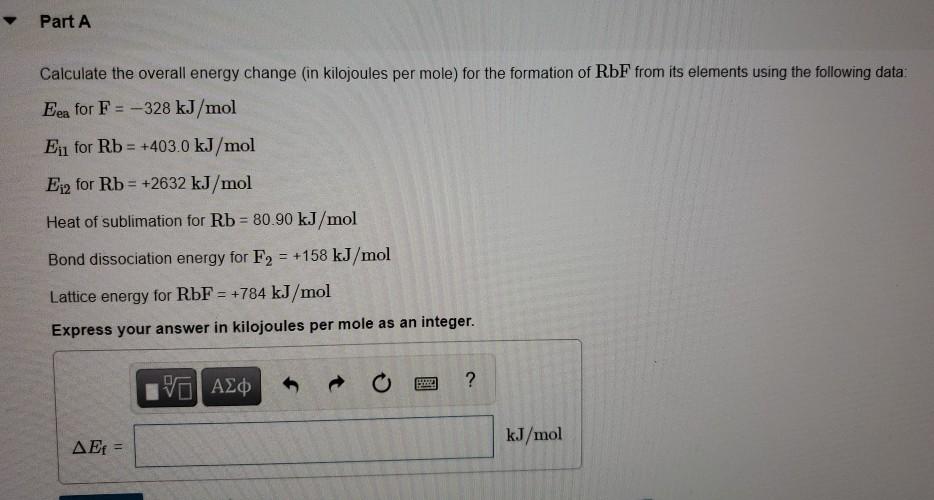
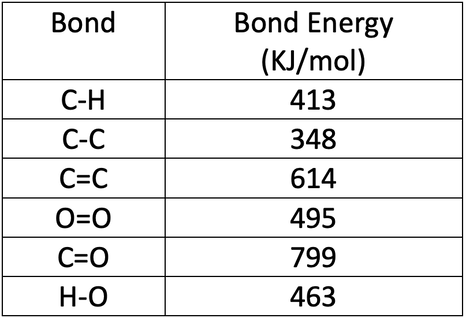
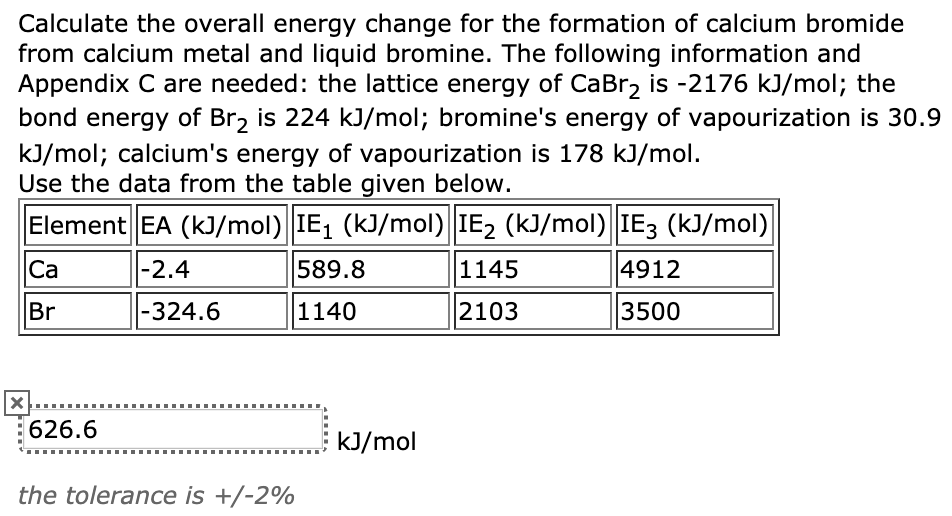
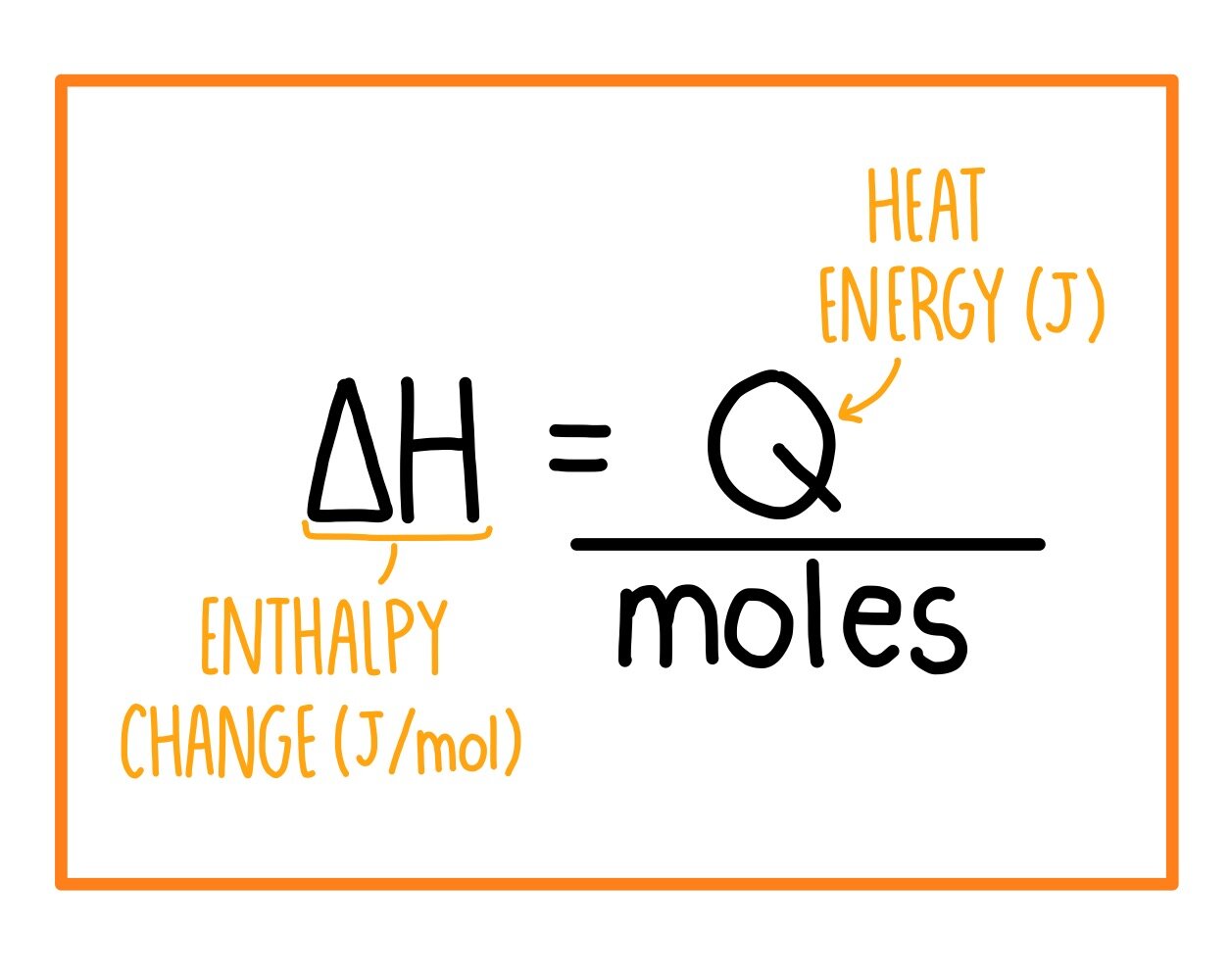Energy Changes" - O Level Chemistry & IP Chemistry Notes by 10 Year Series Author - Chemistry Specialist
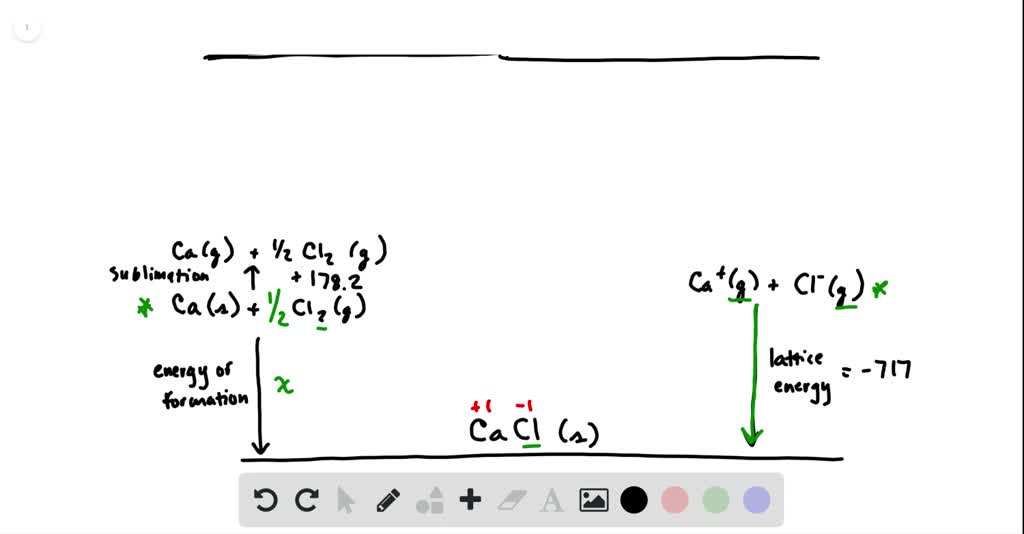
SOLVED:Calculate the overall energy change in kilo joules per mole for the formation of CaCl(s) from the elements. The following data are needed: E ea for Cl(g)=-348.6 kJ / mol Ei 1
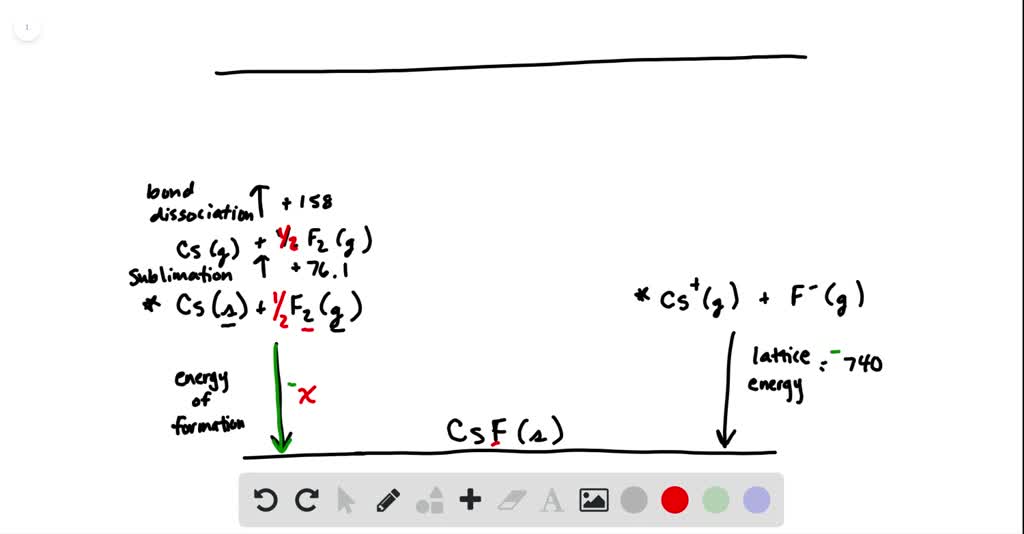
SOLVED:Calculate the overall energy change in kilo joules per mole for the formation of CsF(s) from its elements using the following data: Eea for F(g)=-328 kJ / mol Ei 1 for Cs(g)=+375.7
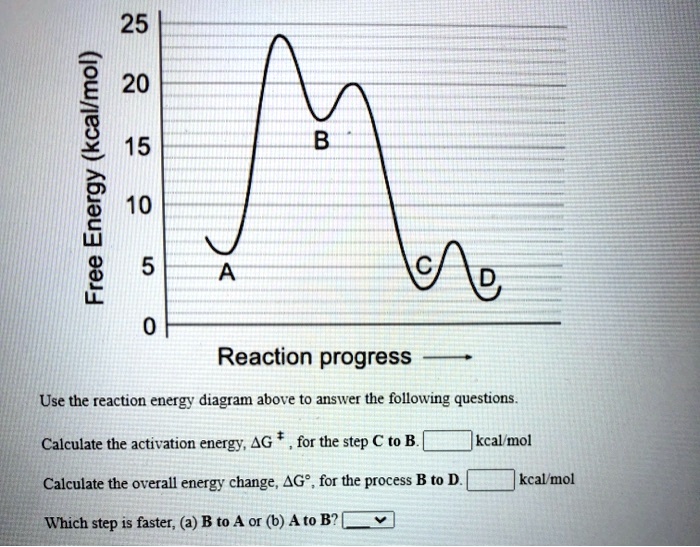
SOLVED: 25 20 (kcallmol) 15 Energy 10 Free 5 0 Reaction progress Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, AG for the step C

Identifying the Energy Change of Reaction from Reactants to Transition States to Products | Chemistry | Study.com